Ester Muniz Twitter
Ester Muniz Twitter - Exploring a Chemical Name
It's interesting, isn't it, how certain phrases or names just seem to pop up and catch our eye, like perhaps "Ester Muniz Twitter." You might wonder what that means, or why it’s a topic of conversation. Well, sometimes, the very words we use, even seemingly simple ones, have more layers than we first think.
You know, words can be a bit like puzzles, so. They carry different meanings depending on the setting, or the way we use them. The word "Ester," for instance, might make you think of a person's name, which is perfectly natural. But, actually, it also has a pretty specific and quite fascinating meaning in the world of science, particularly in chemistry.
So, we're going to take a closer look at what an "Ester" truly means from a scientific point of view. We’ll explore what these chemical compounds are all about, how they are put together, and what they are used for, you know, just to give us a full picture. It’s a way of understanding the term "Ester" more completely, and perhaps seeing how a name, or even a scientific concept, might find its way into discussions online, perhaps even related to something like "Ester Muniz Twitter."
Table of Contents
- What Exactly is an Ester in Chemistry?
- How does the chemical ester connect to "ester muniz twitter"?
- How Are Esters Put Together?
- What are the main parts of an ester, like those you might find in discussions around "ester muniz twitter"?
- What Are Esters Good For?
- How do esters get their names, a bit like how we identify profiles on "ester muniz twitter"?
- Are All Esters the Same?
- What makes some esters stand out, perhaps sparking interest on "ester muniz twitter"?
What Exactly is an Ester in Chemistry?
When you hear the word "Ester," you might think of a person, but in chemistry, it points to a particular kind of chemical building block. Basically, an ester is a type of compound, which is a substance made when two or more different chemical elements join up. This specific kind of compound comes from an acid, either one that's organic, meaning it contains carbon, or one that's inorganic, which usually means it doesn't. So, in a way, it's a sort of chemical offspring, if you can picture that.
The way an ester comes into being is rather interesting. It happens when a hydrogen atom, which is a tiny piece of the acid, gets swapped out. This hydrogen atom is typically found in a special part of the acid molecule called a hydroxyl group. A hydroxyl group is a small collection of atoms, specifically one oxygen atom and one hydrogen atom, joined together. So, that little hydrogen piece from the hydroxyl group gets replaced by something else, and that's how an ester is formed. It’s a pretty fundamental chemical change, really, and it means the acid has been altered in a very specific way. You know, it’s like changing one small part of a recipe to get a completely different dish.
Esters are also described as a class of organic compounds that have a particular reaction when they meet water. When an ester and water get together, they actually break apart. This breaking process creates two new things: an alcohol, which you might recognize as a type of chemical often used in various products, and either an organic acid or an inorganic acid, depending on what the original ester was made from. It's a sort of chemical undoing, if you will, where the ester returns to some of its earlier components. This property is quite useful for chemists, as a matter of fact, because it allows them to manipulate these compounds for different purposes.
How does the chemical ester connect to "ester muniz twitter"?
It's fair to wonder how a chemical compound could possibly relate to a phrase like "ester muniz twitter." Well, in this context, the connection is more about the word "Ester" itself rather than any direct link between a person and a chemical. You see, a word can have multiple meanings, or be used in different ways. So, while "Ester" can be a name, it also has this very precise scientific definition. It's a bit like how the word "apple" can mean a fruit or a brand of computer, honestly. Both are valid, just in different settings.
When we see a phrase like "ester muniz twitter," it could simply be that "Ester" is someone's name, and that person is active on Twitter. There's no hidden chemical meaning there, you know. But by exploring the chemical side of "Ester," we just broaden our general knowledge of the word. It helps us appreciate how language works, how a single word can represent completely different concepts. So, while "Ester Muniz" likely refers to a person, understanding the chemical "ester" gives us a fuller picture of the word itself, which is pretty cool, if you ask me.
This exploration just highlights how words can have a sort of dual existence. On one hand, they're personal identifiers, like names that people use to present themselves online. On the other hand, they're precise labels for scientific concepts, like the compounds we're talking about here. So, if you ever see "ester muniz twitter" trending, you might just have a quiet chuckle to yourself, knowing the other meaning of "Ester" and how different contexts shape our understanding. It's a reminder that language is very rich, and sometimes, words are more than what they seem on the surface, you know, kind of like a hidden layer.
How Are Esters Put Together?
The vast majority of esters that chemists work with, and that you might encounter in various products, come from a specific kind of acid called a carboxylic acid. These are, in some respects, the most common type of ester out there. A carboxylic acid has a particular grouping of atoms that makes it unique, and it’s this grouping that plays a key role in forming the ester. So, when chemists talk about esters, they're often referring to these ones that have their origins in carboxylic acids, basically because they're so prevalent.
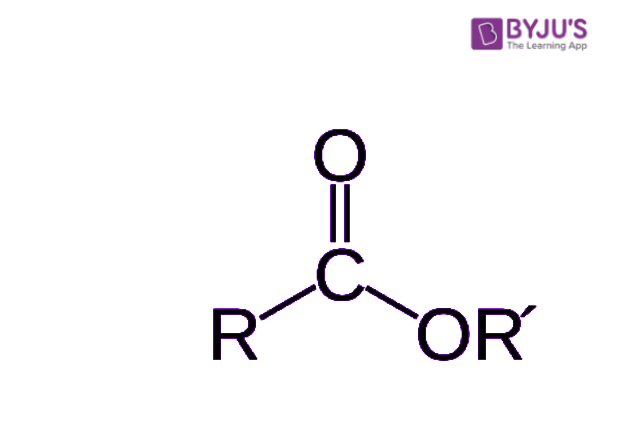
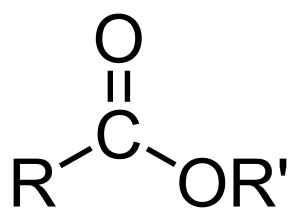
To really get a sense of how an ester is structured, it helps to picture its general arrangement. Imagine a central part where carbon, oxygen, and other atoms are linked up. In an ester, there's a carbon atom that's double-bonded to one oxygen atom and also single-bonded to another oxygen atom. This second oxygen atom, the one with a single bond, then connects to yet another carbon atom. This arrangement is quite distinctive, and it's what gives esters their unique chemical identity. It’s like a very specific pattern that repeats, you know, a sort of signature.
Think of it like building with LEGO bricks. You have specific pieces that fit together in a certain way. For an ester, you always have that core structure involving the carbon and two oxygen atoms, and then other bits attach to it. It’s this consistent internal structure that defines an ester, no matter how big or small the molecule might be. So, when you look at a diagram of an ester, you'll always spot that particular arrangement of atoms, which is pretty neat when you think about it. It's a clear fingerprint for these compounds, honestly.
What are the main parts of an ester, like those you might find in discussions around "ester muniz twitter"?
Every ester molecule, in a way, has a couple of main sections that come from its parent molecules. One part comes from the acid it was made from, and the other part comes from the alcohol involved in its creation. It's a bit like a combined identity, you know, where both parents contribute something. This dual origin is what gives each ester its particular set of qualities and behaviors. So, if you were to break an ester apart, you'd find these two distinct pieces.
The "acid part" of the ester is essentially what's left of the carboxylic acid after it loses a hydroxyl group. And the "alcohol part" is what's left of the alcohol after it loses a hydrogen atom. These two pieces then join up, forming the ester and releasing a molecule of water in the process. This joining is a pretty common type of chemical reaction, actually, and it's how many organic compounds are formed. So, when you look at an ester, you're really seeing a chemical marriage of an acid and an alcohol, which is pretty cool.
When you consider a phrase like "ester muniz twitter," you're dealing with different kinds of "parts" too. You have "Ester" as a potential personal name, "Muniz" as a family name, and "Twitter" as a platform. Each piece contributes to the overall meaning of the phrase, just like the acid and alcohol components contribute to the overall nature of a chemical ester. It's a very rough comparison, of course, but it helps illustrate how different elements come together to form a complete concept, whether it's a chemical compound or a trending topic online. It's about how individual pieces create a larger whole, you know, pretty much always.
What Are Esters Good For?
Esters are pretty versatile compounds, and they have some really interesting uses in our daily lives. One of their most common and widely recognized jobs is in the flavor and fragrance industry. Many of the pleasant smells and tastes we experience, from fruits to perfumes, are actually due to the presence of various esters. They are, quite literally, the chemical essence of many delightful aromas and flavors. So, next time you smell a banana or a pineapple, you're probably getting a whiff of an ester, which is pretty neat.
Because they are so good at carrying scents and tastes, esters are used to create artificial flavors for food and drinks, and to give perfumes and other scented products their distinctive notes. They can mimic the smell of apples, pears, strawberries, and many other natural things. This makes them incredibly valuable for manufacturers who want to create consistent and appealing products. It’s like they’re the little architects of sensory experiences, you know, building up those smells and tastes bit by bit.
Beyond flavors and fragrances, esters have other roles too. Some are used as solvents, meaning they can dissolve other substances, which is useful in paints, varnishes, and other industrial applications. Others might be found in plastics or as components in medicines. So, while their role in making things smell and taste good is perhaps the most famous, their contributions stretch across many different areas of chemistry and manufacturing. They're quite the busy little molecules, honestly, doing a lot of behind-the-scenes work.
How do esters get their names, a bit like how we identify profiles on "ester muniz twitter"?
Just like people have names that help us identify them, chemists have a system for naming esters so they know exactly which compound they're talking about. The names for esters typically include prefixes, which are small parts added to the beginning of a word, that tell you about the lengths of the carbon chains in the molecules. These prefixes give you clues about how many carbon atoms are in different parts of the ester, which is really important for distinguishing one ester from another. It's a bit like a chemical ID card, in a way.
There are two main ways chemists name esters. One is using common names, which are often simpler and more widely known, especially for very familiar esters. The other is using a more formal system called IUPAC nomenclature. IUPAC stands for the International Union of Pure and Applied Chemistry, and they have very strict rules for naming compounds to ensure everyone uses the same, unambiguous name for every chemical. This formal system is very precise, and it helps scientists around the world communicate clearly about their work. It's like having a universal language for chemical structures, you know, for clarity.
For example, if you see "ethyl acetate," that's an ester. "Ethyl" tells you about one part of the molecule, and "acetate" tells you about the other. It’s similar to how on "ester muniz twitter," "Ester" is a first name and "Muniz" is a last name, helping to pinpoint a specific person. While the chemical naming system is much more structured and detailed than human names, the basic idea of using specific labels to identify unique entities is quite similar. It's all about clear identification, whether it's a molecule or a profile online, you know, just to be sure.
Are All Esters the Same?
No, not all esters are the same, not by a long shot. While they all share that basic chemical structure we talked about,
- Exposed Twitter
- Kevin Oconnor Twitter
- Chimocurves Onlyfans Leak
- Siarly Twitter
- Fidan Atalay If%C3%A5%C3%BFa
Ester - Definition, Structure, Esterification along with Properties & Uses
Ester @ Chemistry Dictionary & Glossary
Ester Definition, Examples And Facts | Chemistry Dictionary